
Regulatory Inspection Readiness: A Proactive Approach to CAPA and Compliance
Inspection readiness tips: Learn how to manage fallout and improve with CAPA for better regulatory compliance.
QbD » Blog

Inspection readiness tips: Learn how to manage fallout and improve with CAPA for better regulatory compliance.

Even with the European Commission’s proposal for extending the transitional period for IVDs, manufacturers should start building compliant Technical Documentation now. This article highlights the importance of proactive action despite the proposed delay.

Explore how Environmental Risk Assessment (ERA) helps ensure medicines heal us without harming the planet, balancing healthcare advances with ecological preservation.
The European Commission proposes extending the deadlines for complying with the new In Vitro Diagnostic Medical Devices Regulation (IVDR). This article explores the reasons behind the extension and what it means for manufacturers.
Discover how to navigate compliance with Article 117 of MDR for drug-device combinations. Ensure correct implementation with essential guidance.

Discover how Patient Support Programs (PSPs) and Pharmacovigilance enhance patient care, ensuring safety and compliance throughout the healthcare journey.

Explore the FDA’s final rule integrating ISO 13485:2016 into QMSR for medical devices. Understand impacts on manufacturers and how to comply by 2026.

This article explores why staying on track for IVDR compliance is still the best strategy for medical device manufacturers.
Discover what is required to declare equivalence under the MDR for medical devices in the EU.

Gain an insightful understanding into how the MHRA inspect the Pharmacovigilance services of an MAH for post-authorised products.

Explore how the AI Act impacts MedTech and Life Sciences, and how the QbD Group can guide you through its complexities.
We are pleased to share this meaningful article by two of our senior RA IVD consultants, Pieter Bogaert and Anne Paulussen, recently featured in RAPS

The Medicines and Healthcare Products Regulatory Agency (MHRA) recently announced the upcoming launch of the RegulatoryConnect portal, a groundbreaking step forward in regulatory management. During

The new ICH Q2(R2) is a complete revision of the guideline to include more recent application of analytical procedures and to align content with ICH Q14 “Analytical Procedure Development”.

The United States maintains a robust regulatory foundation for Digital Therapeutics (DTx), ensuring that they are supported by stringent regulatory measures, including clinical evidence and data protection management. Learn more here.

Discover how to create a GMP-compliant stability protocol for pharmaceuticals, ensuring product safety and efficacy throughout its shelf life.

In this blog post, we delve into the intricacies of Digital Therapeutics (DTx) regulation in the UK, especially in light of Brexit in January 2020.

In celebration of International Women’s Day, with ‘Inspire Inclusion’ as the central theme for 2024, we sat down with some of QbD Group’s power ladies.

This blog underscores the critical nature of regulatory compliance in the pharmaceutical industry. It stresses the importance of senior management’s commitment despite significant costs. Key elements like Corrective Action Preventive Action (CAPA) plans are highlighted, especially with the trend towards computerized solutions. Non-compliance risks severe financial consequences, necessitating proactive measures. Effective compliance programs encompass written procedures, designated personnel, training, communication, and monitoring. Compliance is increasingly seen as a strategic advantage rather than just a cost-saving measure.

Explore how to tackle current and upcoming toxicology challenges in the pharmaceutical industry, ensuring product safety and environmental responsibility.

In this blog post, we delve into the DTx regulations of Belgium and France, focusing on their reimbursement strategies and providing a glimpse into how other EU member states fare in comparison.

Explore the crucial role of Pharmacovigilance (PV) audits in ensuring drug safety and compliance, the process, and the challenges faced.
Discover what is required to maintain marketing approval for legacy devices in the EU.

When it comes to the regulation of Digital Therapeutics (DTx), Germany is pioneering new paths for its adoption. This blog post delves into the intricacies of Germany’s approach to DTx regulation.

Quality Audits are the backbone of compliance and improvement. From risk-based planning to meticulous execution and compliance review, each step ensures organizational excellence. Embracing a PDCA (plan, do, check, act) life cycle approach streamlines processes and fosters consistency.

Quality and Regulatory Affairs: Discover the key elements of Quality Management Systems in the pharmaceutical industry for compliance and success.
For DTx manufacturers targeting the European market, compliance with regulation (EU) 2017/745 (MDR) is mandatory. Let’s delve into this regulatory aspect within the EU.

Continuous manufacturing in the pharmaceutical industry enhances efficiency and product quality. Learn about CM’s implementation, control strategies, and ICH Q13 guidelines.

Explore the rise of Digital Therapeutics (DTx), covering its regulatory landscape, reimbursement perspectives, applications, and the WHO’s vision for digital health. Delve into how DTx bridges healthcare gaps and the future of digital health.

Learn key insights to meet the extended MDR/IVDR compliance deadlines in time and ensure your device’s market access and patient safety.

Explore the MHRA’s new International Recognition Procedure (IRP) for medicines post-Brexit, and learn how to navigate regulations and the IRP’s two assessment routes for streamlined medicine registration in the UK.

Discover what is required to obtain and maintain marketing approval for Software Medical Devices in the EU.

Explore the crucial steps for obtaining CE approval for your medical device under MDR. Learn more about the key aspects of conformity assessment tailored to each device class.

On the path to qualifying a new GMP facility? Learn about frequent pitfalls and the best practices we recommend to handle them.

Qualification and validation projects play a pivotal role in guaranteeing that pharmaceuticals, medical devices, and ATMPs meet stringent regulatory requirements. Learn more about QbD Group’s Project Management Process here.

Are you a medicinal product manufacturer looking to import into the EU in line with EU-GMP import regulations? Be sure to read this article.

On 22 May 2023, Irdeto and QBD Group sat down to discuss the developments of the medical device regulatory landscape. Read the interview here.

In this blog post, we explore Computer Software Assurance (CSA), a modern alternative to traditional Computer System Validation (CSV), focusing on its crucial role in the pharmaceutical industry. Discover the key differences between CSA and CSV, the initial steps for transitioning from CSV to CSA, and why the CSA approach is increasingly important for ensuring system reliability, security, and compliance in drug development and manufacturing.
Explore the essentials of plasmid manufacturing and regulations for applications like gene therapy and vaccine development.
Oncolytic viruses and cellular immunotherapy are creating significant new potential in the fight against cancer. Learn more about the working mechanism, risks, and specific biosafety requirements to be considered for oncolytic viruses.

Discover the future of pharma: enhancing efficiency and cutting costs with paperless validation. Learn how to navigate the path to agility and innovation in the digital era.

In this blog post, we delve into the world of GMP-compliant design for climate chambers, unraveling the vital role they play in the stability testing of pharmaceutical products.

In this blog post, we address key regulatory guidelines for stability testing to ensure high-quality products meet regulatory requirements and patient needs.

In this blog post, we would like to share with you the different steps and required documents for the execution of the systematic literature review for the clinical evaluation of your medical device.
In this blog post, we will explore the various factors that influence the stability of medicinal products and the parameters that need to be considered during their storage.

AI, machine learning, blockchain, big data, and other buzzword technologies are making inroads into the biopharmaceutical sector, improving quality, traceability, and efficiency. Read more about how digital health is changing pharma here.

Discover the latest key changes in cleanroom qualification regulations, exploring the impact of the different test phases (qualification, classification, requalification, monitoring) with a focus on risk assessment.

Discover the importance of software validation in clinical trials. Learn how it applies to GCP and GMP systems. Stay ahead in the digital era.

In this blog post, we would like to share with you the definitions, time points, and resources required to execute a systematic State of the Art (SOTA) literature review as required.

In this blog post, we will delve deeper into the significance of stability testing in pharmaceutical development.

Discover how Pharma 4.0 is revolutionizing pharmaceutical manufacturing. Learn about the benefits, challenges and key components and how it improves efficiency, quality and patient safety.

In this blogpost, we are sharing our experience on the common gaps and hurdles that manufacturers are facing when undertaking this Systematic Literature Review (SOTA).

This article addresses the latest revision of EudraLex Volume 4 Annex 11 and provides a checklist for compliance. Download it now.

The past couple of years have borne witness to pivotal shifts in clinical trial approvals within the UK and EU. With the UK implementing the Combine Review system and the EU transitioning from a previous Directive to the EU Clinical Trials Regulation, both regions are aiming for enhanced efficiency, streamlined processes, and heightened transparency. Learn more here.

The EU Commission is proposing a general reform of pharmaceutical legislation. Read more about what this means for patients and the industry.

Maintaining computerized systems during the operational phase is critical to the proper functioning of organizations. In this article, we explore some of the best practices for doing so.

Explore the field of medical writing and the different types of medical writing projects in Healthcare. QbD Clinical is happy to help!

Struggling with CSV validation and testing automation? Tired of re-validating at every sprint? Discover expert tips to reduce time, and budget, and increase validation coverage in our latest blog post on automated software testing for the GxP world.

In this blog post, you will learn more about analytical method validation and the criteria to consider when validating your analytical method.

Although manufacturers will have more time to prepare their technical files for the MDR transition, it is wise not to delay implementation until the last minute. Learn how to plan MDR compliance for your medical device here.

The UK government introduced the Innovative Licensing and Access Pathway (ILAP) in 2021 to give patients quicker access to cutting-edge treatments and therapies by offering a streamlined approach to licensing and regulatory processes. Learn more about ILAP in this blog post.

QbD Group Ltd. attended the MHRA GMDP symposium, a highly anticipated annual event that brings together stakeholders in the pharmaceutical industry to discuss the latest trends and developments. Discover our insights here.

Want to introduce robustness into your software validation activities? In this article, you will learn what the Agile model is and how to use the GAMP 5 framework for agile development.

The EU GMP Annex 1 Manufacture of Sterile Medicinal Products was revised in 2022. In this blog post, we will provide a summary of the changes and our initial insights several months after the revision.
Annex XVI of the MDR EU 2017/745 lists products without an intended medical purpose that now fall under the MDR. In this blog post, you will learn more about the contents and requirements of the annex.

The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) is developing a new IT system to streamline submissions related to product licensing and process licensing. The first release of the system is expected by the end of 2023, with subsequent releases incorporating wider elements such as medical devices and import notifications. An agile delivery model will enable continuous improvement based on user feedback.

Regulatory agencies impose internal audits and supplier audits in the pharmaceutical and medical device industries to ensure compliance with Good Manufacturing Practices and ISO standard requirements. These audits help identify potential problems that may affect product quality, efficacy or safety, and provide a mechanism for implementing corrective actions to minimize public health risks. In this blog post, we’ll discuss 7 key areas to focus on during internal or supplier audits.

Learn 8 logical steps to take prompt action when national competent authority inspection reports, EIRs, FDA Forms 483 or warning letters show non-conformities. Failure to act can have crippling consequences.

In celebration of International Women’s Day, we sat down with Elly De Bruyn, Chief Human Resources Officer (CHRO) at the QbD Group, to talk about her experiences and views as a power lady in the life sciences industry.

Learn how digital health is transforming clinical trials. Explore the benefits and challenges of implementing digital technologies.
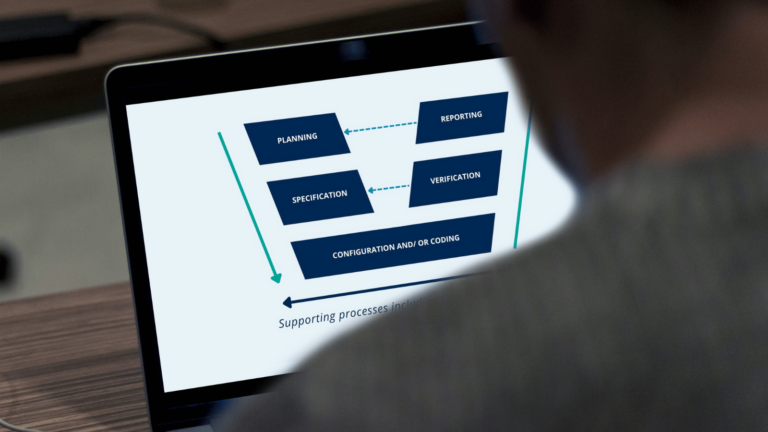
Want to introduce robustness into your software validation activities? In this article, you will learn what the GAMP 5 V-model is and the key steps within it.

This blog post provides an overview of essential documents required during medical device clinical investigations in accordance with ISO14155:2020.

The European Parliament has approved a proposal to extend the transitional deadlines for certain medical devices under the MDR to ensure availability of devices whose certificates have expired or will expire before 26 May 2024. Manufacturers must adhere to specific conditions to comply with the new transition periods. Learn more here.

Are you about to qualify your laboratory equipment? If so, here are the key considerations and challenges to consider.
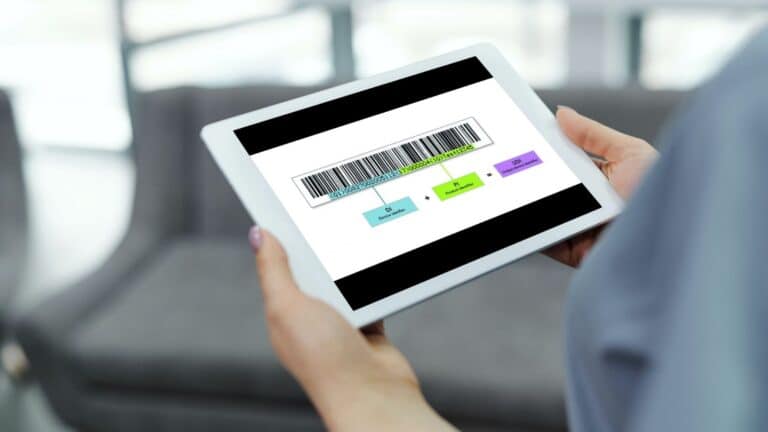
Each individual medical device must have a Unique Device Identifier (UDI) with its carrier (barcode or QR code). But what about medical device software? Read the answer in this article!

Can the computerized system validation used in pharma be applied to non-product medical device software? In this article, we compare ISO 80002-2 with GAMP5 to find out.

In 2023, the IVD industry will continue to dominate the medical technology market due to rising demand for early diagnosis, treatment and point-of-care testing. Product trends include the accelerated consumerization of diagnostics, the rise of telemedicine and digital health, the growth of devices enabling personalized medicine and the emergence of AI and robotics-powered medical devices. Medical device manufacturing will undergo changes in the computerized system validation process due to the increased focus on critical thinking by GAMP5 and FDA.

In this article, you will learn a structured approach to ensure regulatory compliance and proper operation of your computer systems, throughout their life cycle.

December is often symbolized as the ‘Month of Giving’ as it revolves around giving something back to society, helping others and spreading joy. Supporting good causes is one way we put our company value ‘joy’ into practice at the QbD Group. Let’s highlight a few of them!

Artificial intelligence (AI) and machine learning (ML) are on the rise in the medical device and IVD industry. Learn more about the current landscape and regulatory framework here.

Learn more about the importance, benefits, and key challenges related to Annual Product Quality Review in Pharma (APQR/PQR).

Job fair season is upon us! Whether you’re looking for your first job or a new work adventure, we can all agree that applying for a job comes with a healthy dose of nerves. To make sure you are the best version of yourself, preparation is key! Want to succeed in your next interview and land your QbDream job in consulting? Put your foot in the door and kick it wide open with these 5 tips!

Bringing a Medical Device to market involves many hurdles, including design and development. In this blog post, you will learn more about the importance of Design Transfer and Process Validation in medical device development.

Today, on Nov. 14, 2022, we proudly received the Baanbrekende Werkgever ’23 certificate for the second year in a row, representing our commitment to put our employees at the center of our policies around hybrid work and mobility.

Digital health is on the rise, but what is digital health exactly? In this blog post, we’ll explore the landscape, key technology pillars and future opportunities.

21 CFR Part 11 regulates the use of electronic records and signatures in pharma and medical devices. Want to comply? Read more below and download our 21 CFR Part 11 compliance checklist.

Discover 5 reasons to engage with an experienced Contract Research Organization (CRO) specialized in medical device studies to support you in your clinical research.

It is important to understand how standards are written and how their references are constructed in order to properly reference them and purchase the correct version for your use.
In this blog entry, we explain the difference between ISO and IEC standards, what harmonized standards are, and how to use the appropriate standard references in your technical documentation. We also give you a hint on how to make sure you have identified all applicable standards for your products.

Want to learn more about the difference between Artificial Intelligence and Machine Learning and/or need insight into the right validation strategy for your AI- or ML-based systems and products? Read more here.

Have you validated your computerized system(s) yet? Then CSV Periodic Reviews are an important next step. Learn more about their purpose and frequency here.

The quality of process gases and gas distribution networks is becoming increasingly important for biotech pharmaceutical processes and ATMP sites. In this blog, we highlight the importance of a qualified process gas and distribution network, even at an early stage of development.

In 2022, ISPE released a new, second edition of the GAMP 5 guide. Read more about this new edition here and/or watch our webinar on demand.

Self-inspections or internal audits are an essential part of your QMS. In addition to determining whether your QMS meets guidelines and standards, you can benefit from them in several ways. They allow you to get to know departments better, explain to people why certain quality processes are necessary, gather useful feedback for further improvements to the QMS, and you have a tool to check your readiness for external audits.
Are you a manufacturer of custom-made medical devices and in need of regulatory guidance? This article summarizes key concerns to consider.
In Belgium, medicine advertising is subject to legislation in order to ensure the rational use of medicines. Each firm must appoint a Responsible Person for Information and Publicity, who checks the conformity of the advertising.

The Medical Device Regulation (MDR) (EU) 2017/745 and In Vitro Device Regulation (IVDR) (EU) 2017/746 introduced a new mandatory role in Article 15: the Person Responsible for Regulatory Compliance or ‘PRRC’. Wondering what the meaning of the PRRC-role is and where you can find the right person? Then read on!

Considering an exciting career as a life sciences consultant, but still in doubt? In this article, we debunk 6 myths about consultancy to help you decide!

Compressed air and other process gases are used in a lot of different steps during pharmaceutical manufacturing. Some examples are the use of compressed air in direct contact with products to clean, aerate, or move them through the processes or the using process gases in fluid pumps that take products through the production and filling processes. Compressed gases, such as nitrogen or argon, can also be used for blanketing or to spray or coat a product. The risks associated with the use of these gases, depend on the amount and type of product contact and based on this risk assessment, a suitable monitoring plan should be in place.

At the QbD Group, we want to take into account the opinion of our employees. Therefore, our Board of Ambassadors was elected to represent the voice of all QbD’ers, within and outside of the group. Thomas Van Looy has been elected as one of our Ambassadors for 2022. Congratulations, Thomas! As an Ambassador, he loves to share his QbD Group experiences. Want to find out more about Thomas’ #QbDream job? Then don’t miss out on the interview below.

Sometimes it is necessary to perform data migration in computer systems, applications, or software. Learn more about the goal, importance and risks here.
Come to see the QbD Group at stand #3G73 at CPHI Conference in Barcelona. And after the conference…Eat & Connect with lifescience professionals at our QbD’s CPHI Networking Drink.